Catalysts play a key role in many industrial processes, as more than 85% of the energy consumed daily by our essential-routine devices is generated by processes that involve them. Recently, it has been found that under visible light illumination, metallic nanoparticles can effectively catalyse a chemical reaction. In this work, we quantify the energy contribution of these illuminated metal nanoparticles to a chemical reaction.
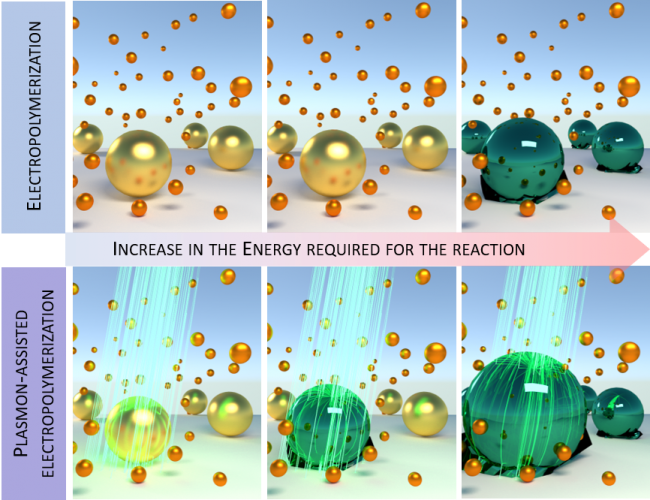
When metallic nanoparticles are illuminated, their conduction electrons are excited in collective oscillations inside the nanoparticle originating what is called a surface plasmon. After excitation, the plasmon has to lose energy (decay process) to return the electrons to their original energetic state. If properly harvested, this released energy can be fruitfully used in many technological processes. Indeed, this is the idea behind plasmon-induced-photocatalysis, a new emerging area where the energy lost by plasmon-decay processes is used for triggering chemical reaction, i.e. to converter a chemical A into the chemical B.
Between all the possible surface-plasmon-decay processes, there are two that have been exploited in plasmon-induced-catalysis: the generation of highly energetic hole/electron pairs and the temperature increase at the interface. Even though both phenomena have been successfully employed as catalysts in numerous chemical reactions, in most of the cases is hard to disentangle the contribution of each of them separately. The other remarkable question behind plasmon-induced-catalysis is associated with the hot-electron/hole pair. These two entities have been theoretically proposed to be as energetic as the energy of the incident light employed for the plasmon excitation. However, this fact has been weakly explored experimentally.
In their work, LCN researchers at Imperial College London addressed the two aforementioned questions by implementing an opto-electrochemical setup. This microscope allowed them to: illuminate a single gold nanoparticle (AuNP) with different CW lasers (different plasmon-excitation energies), determine the AuNP surface temperature (by the photoluminescence properties of illuminated AuNPs) and follow its catalytic behaviour (by the scattering properties of AuNPs using a dark-field microscope). Their results show that the illuminated AuNP can catalyse the chemical reaction under study by using less input energy from external sources. This could help in the future to convert sunlight energy into chemical energy or green fuels by finding catalysts that can perform this energy conversion step efficiently.
Link to research paper: Spectral Screening of the Energy of Hot Holes over a Particle Plasmon Resonance