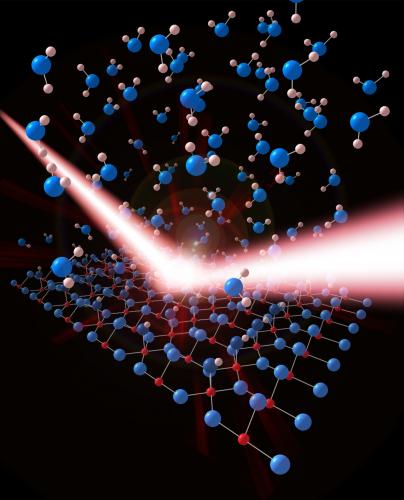
Water is a crucial molecule to have a good understanding of with respect to adsorption on solid surfaces. This is mainly due to its ubiquity and therefore its pivotal role in many scientific processes including catalysis and corrosion protection.
A study involving a multi-institutional collaboration driven by a group of LCN researchers, have just published their results on the interaction of water with a metal oxide, TiO2, in the prestigious Nature Materials journal.
The surface chemistry of water interacting with TiO2 is crucial to many of the every-day practical applications of TiO2, including photocatalytic water splitting. The team of researchers have developed a novel approach to provide the first quantitative structure of a TiO2/liquid water interface, which is a more relevant phase combination for practical applications than the gas-phase water interface with a metal oxide, which to-date has been the most widely studied. Indeed, the structure that the team have identified represents a good model of the interface present in the rutile TiO2 photocatalyst.
Scanning tunnelling microscopy results from UCL and Surface X-ray diffraction measurements taken at the European Synchrotron Radiation Facility (ESRF) in Grenoble were used to elucidate the structure of this model photocatalytic interface at a quantitative level, which has terminal hydroxyls in the contact layer. This model together with density functional theory calculations suggest a mechanism for the way in which these hydroxyl molecules align on the metal oxide surface as a half monolayer, corresponding to a (2 x 1) structure: thought to be through the mixed dissociation of O2 and H2O on a rutile surface containing point defects. Stability of the over-layer was shown to rely on a competition between charge transfer arising from the presence of defects and the surface distortion due to the adsorption of the hydroxyls.
By providing accurate experimental structural data for a model photocatalytic interface, the researchers hope this work paves the way to the investigation of the elementary steps involved in water splitting under operating aqueous conditions. This may ultimately lead to an atomistic-level understanding of the photocatalytic process of water splitting, which would allow the design of more efficient devices.
The full article in Nature Materials: Structure of a model TiO2 photocatalytic interface
Related links:
The London Centre for the Theory and Simulation of Materials