Solid substances are made of atoms or ions. These may be arranged in an ordered pattern, like in a rock salt crystal, or they may be completely disordered, like in window glass. But more common than either extreme is the intermediate situation where order and disorder coexist in the same material. To understand the properties of such partially disordered materials is a significant challenge.
Researchers at the London Centre for Nanotechnology, in close collaboration with researchers at The Paul Scherrer Institute in Switzerland, Edinburgh University, Oak Ridge National Laboratory USA and the Institute Laue-Langevin in France, have now discovered that the partial disorder of ions in a particular material is not random but instead obeys a hidden mathematical rule which determines other aspects of the material’s behaviour. This rule was exposed by scattering neutrons off the ions. Because different ions scatter neutrons to different degrees and because neutrons are quantum particles that interfere like waves, the neutron scattering pattern reflects how the ions are arranged within the material.
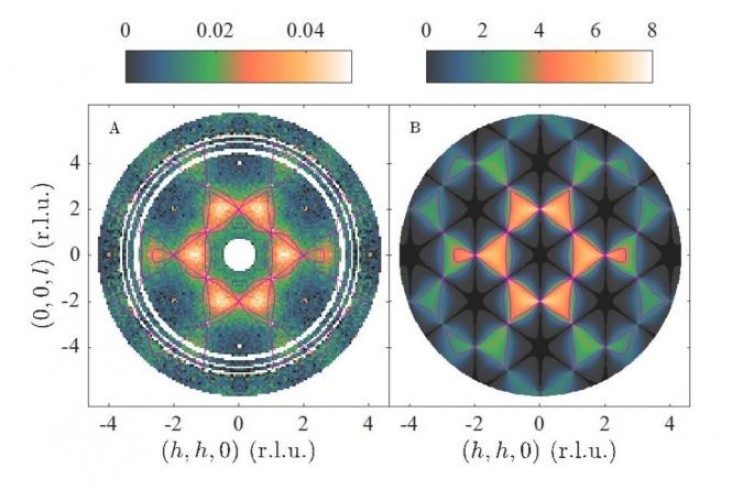
The researchers studied a material consisting of caesium, nickel, chromium and fluoride ions. They found that the nickel and chromium ions are not ordered like a crystal, but instead connect to form closed loops of many different sizes, from the nanometer scale to the millimetre scale. In mathematics the ions are said to have a closed loop topology. It was shown that the closed loop topology of the ions goes on to determine other properties of the material: its magnetism and how all the ions move as the crystal is heated up.
Physicists refer to the closed loop topology as a “Coulomb phase”. This is a reference to the fact that when a closed loop is broken, there is a line that simply terminates, just like the way that electric field lines terminate on electric charges. The French scientist Coulomb famously measured the force between electric charges in the 18th Century. Hence the closed loop topology is like a vacuum from which effective Coulomb charges emerge.

Figure: Neutron scattering pattern of the material CsCrNiF6 which contains a “multiple Coulomb phase”. Left, experiment and right, theory. Analysis of such patterns showed that atomic positions, atomic movements and magnetic properties are all controlled by the same mathematical rule (T. Fennell et al., Nature Physics)
Because the Coulomb phase manifests in several different properties, the researchers named their material a “multiple Coulomb phase”, which represents a new type of partially-disordered matter.
“To describe partially disordered materials is a challenge” explains Professor Steve Bramwell of the London Centre of Nanotechnology. “To have found a hidden mathematical rule that explains so many properties is an exciting development.”
The work is published in Nature Physics.