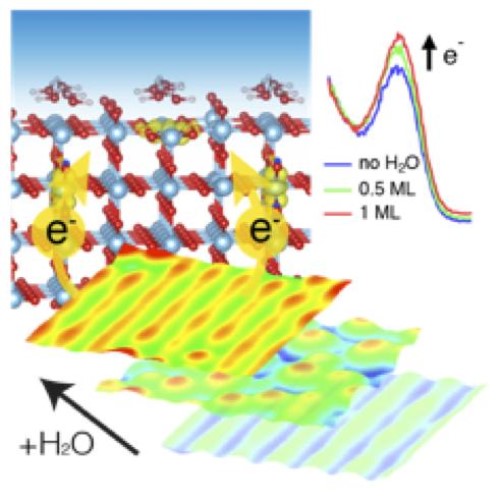
Titania has been the subject of a tremendous amount of research, most of it related to its photocatalytic applications. Central to these studies has been the work that has explored the interaction of water with TiO2 surfaces. Indeed, this is now quite well understood. What has not been investigated so far is the effect of water on the electronic structure, which is examined in the present study.
Led by Prof. Geoff Thornton of the London Centre for Nanotechnology, a multi-institutional team of experimentalists and theoreticians from the UK and US present a low temperature scanning probe and photoemission study that demonstrates that electron polarons are attracted to individual water molecules. Density functional theory calculations show that water stabilises the location of polarons in the top layers of the interface.

Figure: Through detailed studies of adsorbed water nanoclusters and continuous water overlayers, we determine that excess electrons in TiO2 are attracted to the top surface layer by water molecules
Measurements on methanol show similar behavior. Our results suggest that adsorbate-induced surface segregation of polarons could be a general phenomenon for technologically relevant oxide materials, with consequences for surface chemistry and the associated catalytic activity. Moreover a similar effect was observed for a multilayer of water, demonstrating that the phenomenon is also present in realistic environments.
The full article in Journal of Physical Chemistry Letters: Visualization of Water-Induced Surface Segregation of Polarons on Rutile TiO2(110)