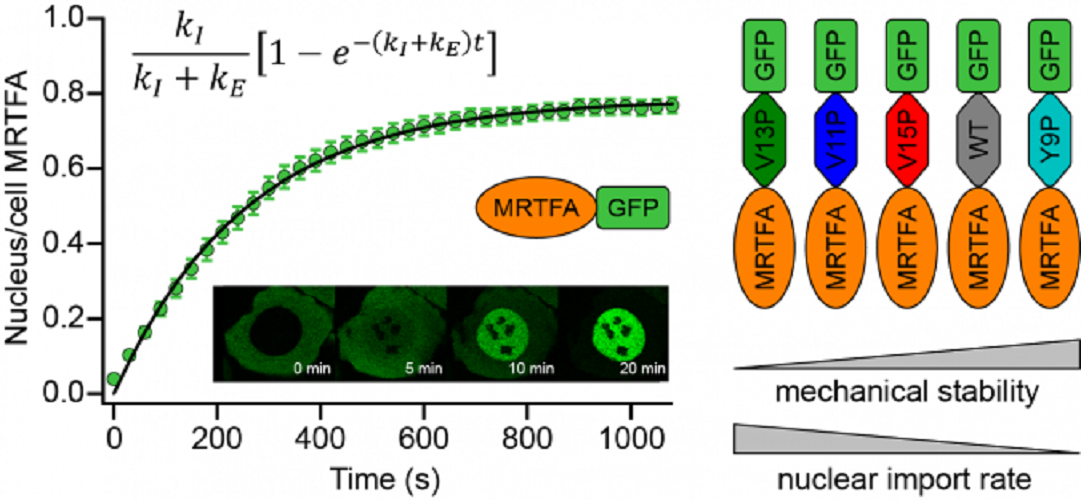
The nuclear pore complex (NPC) is the primary transport gateway for biomolecules to enter and exit the cell nucleus. It has long been known that the passage of proteins through the NPC is highly size-selective, and it was recently shown that the nuclear import rate also depends on the surface chemistry of the translocating cargos – surface-exposed hydrophobic residues massively increase the import rate. LCN researchers at King’s College London have now shown that the mechanical stability of translocating proteins is an additional factor controlling their nuclear translocation.
The researchers conjectured that the mechanical unfolding of translocating proteins, to expose hydrophobic residues buried within the protein fold to the NPC lumen, would increase their nuclear import rate. To investigate this hypothesis, they used a model system in which protein nuclear translocation could be biochemically initiated on-demand and subsequently monitored – fluorescently-tagged MRTFA (myocardin-related transcription factor A) in an osteosarcoma cell line. MRTFA readily translocates to the nucleus upon serum stimulation and the fluorescent tag allows this process to be monitored by confocal microscopy. The mechanical stability of MRTFA was controlled by concatenating it with a range of small protein domains whose mechanical stabilities had been individually characterised using single-molecule force spectroscopy. They observed a clear inverse relationship between the nuclear import rate of chimeric MRTFA constructs and the mechanical stability of the concatenating domains, demonstrating for the first time that the mechanical stability of proteins regulates their nuclear import.
Finally, they considered whether this control over MRTFA nuclear translocation has knock-on effects on gene expression levels and cell function, and found that increasing the mechanical stability of MRTFA downregulates multiple motility-related genes and slows migration in osteosarcoma and breast cancer cell lines. Their approach suggests that modulating the mechanical stability of transcription factors could be a general mechanism to regulate gene expression.
Link to article in Nature Physics: The mechanical stability of proteins regulates their translocation rate into the cell nucleus